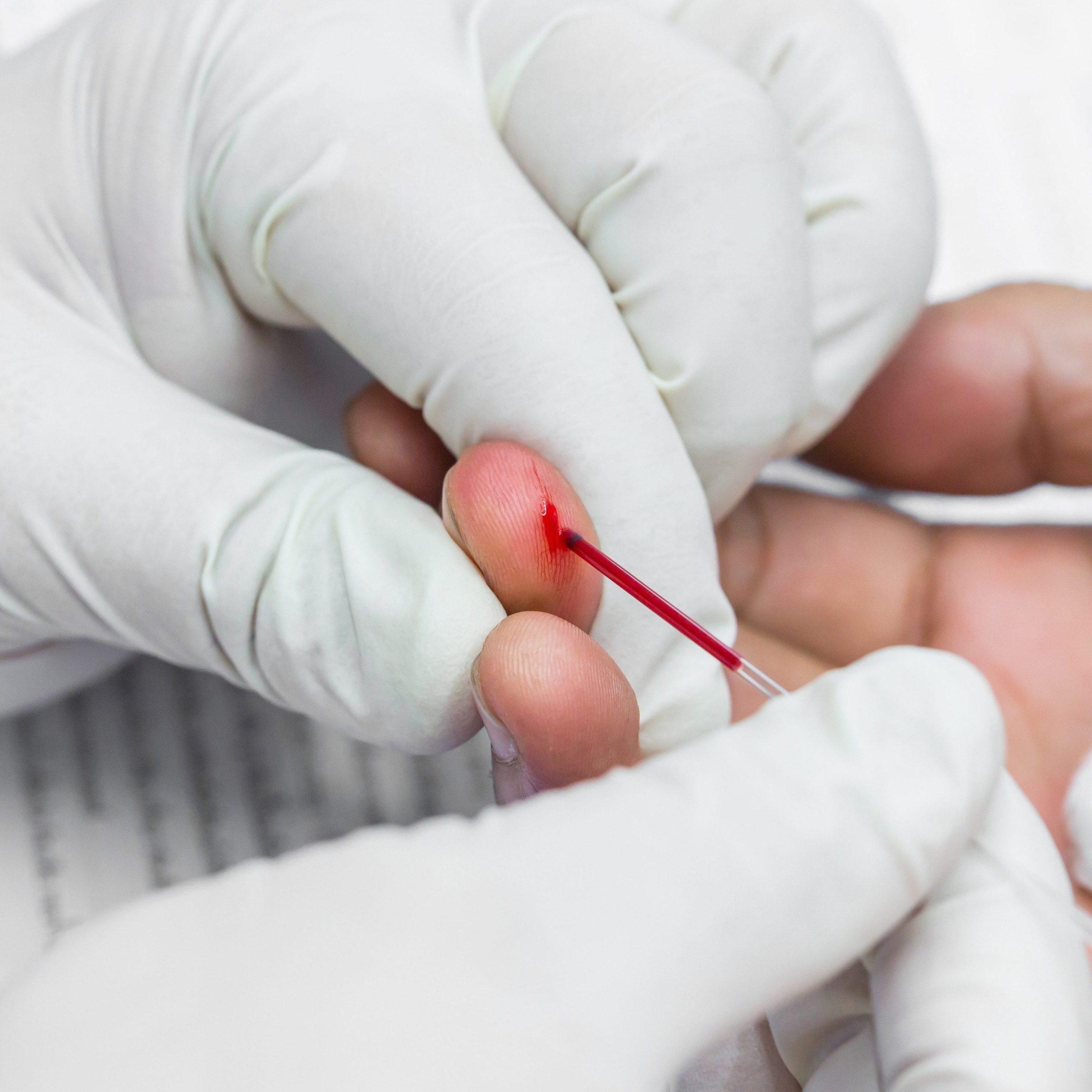
care
For non-emergency situations, a range of POCT supplies comes in stores or online for personal use.
Here at American Screening, we’re your number one supplier of personalpoint of care testing products.
From glucose to DNA to hormone testing, from flu tests to pregnancy tests, as well as pain relievers and healing supplies, we offer quick solutions to your healthcare needs.
Our products are convenient to use, highly effective, and include our top-quality customer support.
Whatever your point-of-care needs are, we can help supply them directly.
Conversely, in emergency situations in a hospital, rapid tests could be given to
However, a lot of people may self-present at a frontline healthcare facility (e.g., an acute care hospital or other emergency care setting, including urgent care clinic, or critical access hospital), without prior notification.
Healthcare facilities should conduct thorough travel history and screening of most patients.
If Ebola disease is a concern, the patient should be promptly isolated in accordance with CDC’s guidance for emergency departments and the local/state health department [PDF – 2 pages] should be consulted to find out if the patient should be referred for ebolavirus testing.
The scoping review identified studies that were conducted in a variety of healthcare settings, in addition to in the
This table represents a consensus framework for considering treatment goals for glycemia, blood pressure, and dyslipidemia in older adults with diabetes.
Consideration of patient and caregiver preferences can be an essential requirement of treatment individualization.
For example, glucose meters and point-of-care hemoglobin A1c tests were created only for monitoring diabetes and should not be utilized for diagnosis or screening.
One nervous about conventional laboratory testing is that folks might not return for treatment if they have to go home and wait for results.
Other examples include such tests as occult blood, ova and parasites, and blood cultures.
Blood cultures may be ordered for a specific time if a bloodstream infection is suspected.
Statin Treatment
CDC will work with the facility to determine proper reporting and handling of specimens from these patients.
If clinical laboratories opt to require supplementary PPE beyond what’s recommended above, this might necessitate additional steps (e.g., staff should be fit tested and medically cleared to wear an N95®respirator).
- or local public health department and then to CDC as directed by the state.
- If a clinician receives test outcomes related to COVID-19 from duplicate specimens that were collected in the same manner and tested with different test methods (e.g., different platforms) or in different CLIA-certified laboratories, the clinician shouldn’t report both results.
- POC testing devices employed remotely enable doctors to see in near-real time necessary information about their patients, referred to as remote patient monitoring.
- Whatever your point-of-care needs are, we are able to help supply them directly.
- However, POCT devices might not be as reliable as laboratory-based drug testings as greater rate of falser positive plus some false negative results may be observed using POCT devices in comparison to laboratory-based urine drug screening using immunoassays and automated analyzers.
However, one study indicates that a possible factor behind the high usage of POCT in France may be the decision to use fewer centralised laboratories, which creates a gap in diagnostics that POCT could fill .
We used the statistical program, R , to summarise categorical data using counts and percentages, and qualitative thematic analysis to recognize common themes from open text data .
However, studies examining the effects of intensive glycemic and blood circulation pressure control to achieve specific targets haven’t demonstrated a reduction in brain function decline.
7.12 rtCGM A or isCGM C can be utilized for diabetes management in adults with diabetes on basal insulin who are capable of using devices safely .
Data may be blinded or noticeable to the person wearing the device.
These devices are not fully owned by the patient—they are clinic-based devices, instead of the patient-owned rtCGM or isCGM CGM devices.
diagnosis across different sites, with studies showing that high rates of errors were observed even in performance of simple RDTs for HIV and malaria15,68.
Quality of testing requires proficiency panels be delivered to all the POC testing sites by way of a national reference laboratory.
Including positive and negative controls with each box of tests will allow all tests to be approved after they reach their destination and before first use.
Recently, nine African countries developed a national system for assuring the caliber of POC testing for HIV69,70.
With data connectivity from each testing site, quality assurance results can be linked to test results at each POC testing site at a centralized database to trigger alerts for corrective action.
Supply chain software may also be linked through these connectivity solutions, avoiding stock-outs.
The scoping review results provide some indication of where POCT is regarded as potentially useful.
Common examples of POC test tools include blood glucose monitors, thermometers, home pregnancy tests and rapid strep tests.
Pharmacy-based Test and Treat empowers pharmacists to execute diagnostic tests within an in-person clinical setting.
This pharmacist-led service increases patients’ access to health care by allowing pharmacists to execute appropriate physical assessments and diagnostic tests also to treat patients predicated on a variety of physical and diagnostic test results.
The requirements for reporting laboratory testing data are designed to inform rapid public health responses.
Reporting requirements usually do not apply to specimens which were collected 2 months prior to the date of testing.
Trending Topic:
 Market Research Facilities Near Me
Market Research Facilities Near Me  Cfd Flex Vs Cfd Solver
Cfd Flex Vs Cfd Solver  Tucker Carlson Gypsy Apocalypse
Tucker Carlson Gypsy Apocalypse  CNBC Pre Market Futures
CNBC Pre Market Futures  Stock market index: Tracker of change in the overall value of a stock market. They can be invested in via index funds.
Stock market index: Tracker of change in the overall value of a stock market. They can be invested in via index funds.  Best Gdp Episode
Best Gdp Episode  PlushCare: Virtual healthcare platform. Physical and mental health appointments are conducted over smartphone.
PlushCare: Virtual healthcare platform. Physical and mental health appointments are conducted over smartphone.  Mutual Funds With Low Initial Investment
Mutual Funds With Low Initial Investment  Jeff Gural Net Worth
Jeff Gural Net Worth  Beyond Investing: Socially responsible investment firm focusing on firms compliant with vegan and cruelty-free values.
Beyond Investing: Socially responsible investment firm focusing on firms compliant with vegan and cruelty-free values.