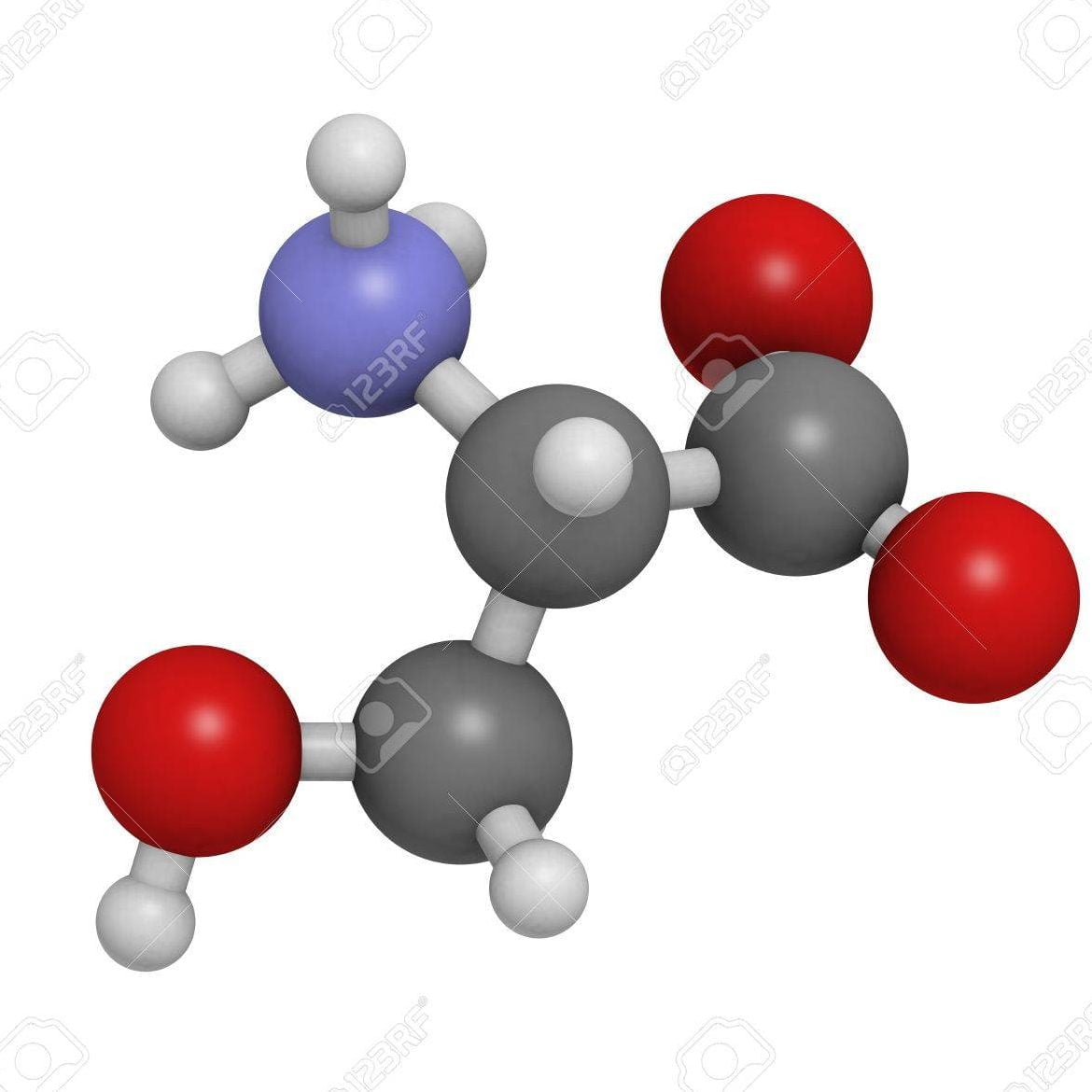
vaccine
Their results showed that this vaccine leads to a considerable reduction in viral load and prevents pneumonia in ferrets after SARS-CoV challenge .
All these results have encouraged subsequent development of adenovirus-based MERS and COVID-19 vaccines.
Viral vector vaccines are recombinant viruses that encode antigens of interest in an unrelated modified virus.
It launched phase 1/2 trials in July 2020, and in September a phase 3 trial with 60,000 participants in Latin America.
J&J has committed 500 million doses of the vaccine to the COVAX initiative for distribution worldwide.
In January, the business announced that the vaccine had an efficacy of 66% in Latin America, 57% in South Africa and 72% in america, with 100% efficacy against severe disease in all trials.
The Existing Vaccine Landscape
Chevalier M.F., Bobisse S., Costa-Nunes C., Cesson V., Jichlinski P., Speiser D.E., Harari A., Coukos G., Romero P., Nardelli-Haefliger D., et al.
High-throughput monitoring of human tumor-specific T-cell responses with large peptide pools.
Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies contrary to the Novel Coronavirus SARS-CoV-2.
Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al.
Zeltins A., West J., Zabel F., El-Turabi A., Balke I., Haas S., Maudrich M., Storni F., Engeroff P., Jennings G.T., et al.
Incorporation of tetanus-epitope into virus-like particles achieves vaccine responses even yet in older recipients in models of psoriasis, Alzheimer’s and cat allergy.
Yang Z.-Y., Kong W.-P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice.
called messenger RNA, or mRNA.
Following the vaccine is injected into a person’s arm, the adenoviruses bump into cells and latch onto proteins on their surface.
Once inside, the adenovirus escapes from the bubble and travels to the nucleus, the chamber where the cell’s DNA is stored.
Reduced antibody responses to the pandemic vaccine after recent seasonal influenza vaccination.
- Now the company has launched a phase I/II clinical trial to judge its safety and efficacy profile in adults .
- SAGE currently recommends its use in line with the WHO Prioritization Roadmap, even if virus variants are present in a country.
- Unlike the company’s first COVID-19
- The 4,000 participants will be equally split, with each half receiving two doses of either vaccine as a way to allow researchers observe whether VLA2001 produces similar levels of antibodies to Vaxzevria.
- The $230 million investment makes CEPI undoubtedly the best funder of research and development on vaccines that
- WHO is also involved with all areas of thwarting the COVID-19 pandemic.
Rather, the high danger comes from genetic recombination events in animals infected with multiple viruses, as known from influenza as genetic shift, the normal origin of influenza pandemics .
In any case, vaccine development should be accelerated to handle future challenges, since new coronaviruses or other microbes may threaten the world’s population.
Unfortunately, most serology tests cannot directly determine if the detected antibodies neutralize the virus, a mandatory parameter if protection is to be predicted.
Upscaling of neutralizing antibody testing is bound because assays that directly demonstrate neutralization of SARS-CoV-2 should be done with the herpes virus itself, that is only safe in highly specialized biosafety level-3 laboratories.
Many groups are suffering from neutralization assays with retroviruses or vesicular stomatitis virus pseudotyped with the SARS-CoV-2 S protein, allowing neutralization assays to be achieved in biosafety level-2 facilities .
A surrogate for neutralization is antibodies specific for RBD, because so many SARS virus-neutralizing antibodies are binding to RBD , although a small amount of antibodies may neutralize SARS without binding RBD .
May I Wait Longer Than Recommended To Get My Second Dose?
School of Medicine at Mount Sinai in NY.
NDV-HXP-S is founded on the Newcastle Disease Virus , that may be grown in large quantities in chicken eggs, similarly to the well-established influenza vaccines which were produced because the 1950s.
Phase 2 trials of this vaccine have begun in January, 2021 in Singapore and the United States.
Phase 1/2 participants produced an immune response similar to those who recover from COVID-19.
Phase 2 trials of the vaccine have just begun with 1,000 participants.
In collaboration with the Lazzaro Spallanzani National Institute for Infectious Diseases it launched a phase 1 trial at the end of July 2020, with subsequent results indicating that participants produced antibodies.
Turkey-based Erciyes University completed a phase 1 trial because of its vaccine candidate, called ERUCOV-VAC, in December 2020, which was followed by a phase 2 trial.
A phase 3 trial were only available in October 2021 to to compare short and long-term immunogenicity of different COVID-19 vaccine combinations against the ancestral SARS-CoV-2 and also different variants of concern.
The development of SARS-CoV protein subunit vaccines was initially surrounding full-length S protein-based vaccines and then later focused on S protein RBD-based vaccines.
None of the SARS-CoV protein subunit vaccines entered clinical trials, however they induced potent antibody responses and protective effects in preclinical models .
Studies have shown that full-length S protein, extracellular domain of the S protein, and trimeric S proteins are immunogenic and may elicit protection against SARS-CoV infection .
However, Kam et al. and Jamue et al. have discovered that triSpike vaccine may also cause Fcγ receptor II (FcγRII)-dependent SARS-CoV infection in human B cells in vitro .
However, S protein RBD-based vaccines can induce high-titer neutralizing antibodies without causing obvious pathogenic effects .
Recent investigations show that the brand new SARS-CoV-2 virus uses a similar strategy for cell entry .
Attachment to host cells occurs via binding of the viral S protein to the angiotensin-converting enzyme 2 , the viral receptor on host cells.
Subsequently, the S protein is primed by host cell proteases, by furin and the serine proteases TMPRSS2 and TMPRSS4, enabling the fusion of viral and cellular membranes and the consequent entry of viral RNA into the host cell .
Weighed against vaccine hesitancy, global vaccine equity and access may be the far bigger hurdle.
There is no quick fix to improve global vaccine supply, which involves issues of reagent supply, qualified manufacturing staff, manufacturing capacity, regulatory hurdles and distribution, even minus the larger context of vaccine nationalism.
One problem that has been discussed prominently is intellectual property.
The vaccine efficacies were 91.3% against COVID-19 infection and 96.7% against severe disease through 6 months of follow-up in a large-scale clinical study .
BNT162b2 is among the hottest vaccines around the globe, in fact it is authorized in 146 countries by August 2022.
The approved vaccines use a range of different platforms (mRNA, viral vector, protein/peptide and inactivated virus).
Comparisons of the partnership between efficacy and neutralizing and binding antibody titres in vitro across various vaccine platforms have been conducted17,18.
These data suggest higher antibody responses to the mRNA vaccines also to the Novavax protein subunit vaccine than to the viral-vectored and inactivated virus vaccines.
Dissecting why different vaccine platforms induce a different quality and/or quantity of immune response will be imperative to develop successful vaccine approaches for future pandemics.
One method of avoid difficulties of comparison between vaccine platforms for future pandemics is always to have pre-prepared trial protocols that could be adopted rapidly by multiple teams.
Contents
Trending Topic:
 Market Research Facilities Near Me
Market Research Facilities Near Me  Cfd Flex Vs Cfd Solver
Cfd Flex Vs Cfd Solver  Best Gdp Episode
Best Gdp Episode  Tucker Carlson Gypsy Apocalypse
Tucker Carlson Gypsy Apocalypse  Stock market index: Tracker of change in the overall value of a stock market. They can be invested in via index funds.
Stock market index: Tracker of change in the overall value of a stock market. They can be invested in via index funds.  Arvin Batra Accident
Arvin Batra Accident  90day Ticker
90day Ticker  CNBC Pre Market Futures
CNBC Pre Market Futures  Robinhood Customer Service Number
Robinhood Customer Service Number  Phil Town Portfolio
Phil Town Portfolio